Innovation Timeline
14 years of breakthrough medical device development - from Tenease to Plantarcure
The development of our product line represents more than technological innovation - it's a story of understanding patient needs and improving quality of life for those suffering from musculoskeletal conditions.
Tenease: The Revolutionary Beginning
Developed by Mr. Ranjan Vhadra, a UK surgeon, Tenease revolutionized tennis elbow treatment. Winner of the 2009 Schroders Innovation competition, it became the gold standard for lateral epicondylitis treatment.
Before Tenease, treatments were limited to rest, injections, or surgery - each with significant drawbacks. Our breakthrough introduced portable, non-invasive low-frequency vibration therapy for home use.
Champion Endorsement
Used by Richard Krajicek, 1996 Wimbledon men's singles champion, to treat his golfer's elbow (medial epicondylitis).


Kneease: Expanding to Joint Care
Building on Tenease's success, we adapted the technology for knee conditions. Drawing from our medical team's expertise in knee and hip joint replacements, Kneease maintained core principles while addressing knee-specific anatomy.
Clinical Insight
While effective for pain relief, we discovered limitations with degenerative conditions like osteoarthritis - the device provided symptomatic relief but couldn't address underlying structural damage. However, it proved valuable for pre and post-operative pain management.
Sciaticalm: Targeting Nerve Pain
Addressing the debilitating pain of sciatica, we developed Sciaticalm to target the sciatic nerve pathway. This adaptation proved highly effective for nerve-related pain relief, using targeted vibration therapy along the lower back and sciatic nerve route.

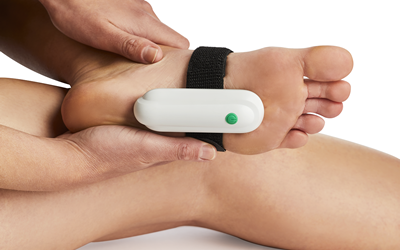
Plantarcure: Advanced Foot Therapy
Our most advanced device addresses plantar fasciitis, adapting vibration therapy specifically for the plantar fascia. Like lateral epicondylitis, plantar fasciitis stems from reduced blood flow to tendon fibres - ideal conditions for our proven technology.
Major Technological Leap
The Future of Pain Relief
Our journey from Tenease to Plantarcure represents more than product evolution - it's healthcare innovation that transforms lives through effective, non-invasive, accessible pain relief.
Smart Connected Devices
IoT integration for treatment tracking and optimization
AI-Powered Therapy
Personalized treatment protocols based on patient data
New Condition Adaptations
Expanding to additional musculoskeletal conditions
Help Shape the Future
What condition would you like us to address next? Let us know and we'll aim to make it happen.
Share Your IdeasClinical Excellence
Each device undergoes rigorous testing, clinical trials, and regulatory approval. Our CE approval for Tenease marked a major breakthrough in bringing hospital-grade technology to home treatment.
Patient-Centric Design
Our devices prioritize patient comfort, accessibility, and effectiveness. Home treatment eliminates expensive medical visits while enabling sustainable long-term condition management.

